Yacht Corrosion
From CruisersWiki
Delatbabel (Talk | contribs) (→Galvanic Corrosion) |
Delatbabel (Talk | contribs) m (→Cathodic Protection (Zinc)) |
||
| Line 53: | Line 53: | ||
===Cathodic Protection (Zinc)=== | ===Cathodic Protection (Zinc)=== | ||
| - | + | As can be seen from the galvanic chart above, zinc as a metal is much more reactive than most metals used in shipbuilding. In fact it is the most reactive metal other than magnesium and a few others not shown on the chart, however neither magnesium nor the other metals are suitable for use as a protection method due to the fact that they cause over-protection, which leads to alkali corrosion of the metal you are trying to protect. | |
| + | (In fact magnesium is suitable for use as a protective anode, however only in fresh water). | ||
| + | |||
| + | So in simple terms, cathodic protection involves turning the metal that you want to protect into a cathode of an electrical cell, while the zinc (also in the water) becomes an anode. In effect you encourage the galvanic corrosion of the zinc and in exchange you will prevent galvanic corrosion of the protected metal. | ||
| + | |||
| + | In order for this to work, the zinc and the metal you are trying to protect must be: | ||
| + | |||
| + | * In the same body of water -- zinc on the outside of your boat will not protect metal fittings in a wet bilge; and | ||
| + | * Electrically connected, outside of that body of water. For example, the zinc should be welded to a steel hull, or in the case of protecting thru-hulls in a fibreglass or wooden boat, connected electrically using a bonding circuit in the inside of the boat to the metal that it is there to protect. | ||
| + | |||
| + | There are many ways of sizing zinc anodes for a particular sized hull but the most common involves a simple formula involving the length (and wetted hull area) of the boat which will give you a size in kg of the zinc anode requirements. | ||
===Install Bonding Circuits=== | ===Install Bonding Circuits=== | ||
Revision as of 11:27, 5 March 2009
Contents |
Corrosion on Yachts
Background
Simply enough, if you mix different metals and add salt water, you will get corrosion. That's a fundamental law of physics. It's also a fact of life that boats live in salt water, and often contain many different metals. It's a wonder that some of them still float!
The problems of corrosion fall into these broad categories:
- Corrosion of steel, and to a lesser extent aluminium and alloy hulls.
- Corrosion of external hull fittings, including stern tubes, exposed propeller shafts, propellers, and other exposed metal parts underwater.
- Corrosion of internal metal fittings such as electrical and electronic components.
Metal Parts underwater
Metal parts underwater are particularly susceptible to corrosion, both galvanic and stray current corrosion. This includes steel hulls which must be protected both by using suitable cathodic protection systems as well as a coating system.
Types of steel, stainless steel and other metals and alloys
Causes
Galvanic Corrosion
Galvanic corrosion is caused by two dissimilar metals, with different galvanic potentials, joined by two separate electrical paths including an electrolytic path (e.g. salt water, which is a liquid electrolyte). Similar to what happens inside a lead-acid battery, although on a much slower scale, an electrical potential (voltage difference) develops between the two metals when an electrolyte can pass ions between them, and all that is needed is an electrical path between the metals outside of the electrolyte solution, and you have an electrical current as well as corrosion happening.
There are many sources of this, including:
- Two dissimilar metals being immersed in salt water, with an electrical path between them. e.g. a propeller shaft and a bronze thru-hull fitting, or a bronze propeller and a steel hull.
- Small drops of water (especially salt spray) making a connection between two electrically connected metals aboard the boat. For example, a small drop of salt spray getting between an exposed copper wire and a steel bolt, or into a small crack between an aluminium mast fitting and the steel base that it is welded to.
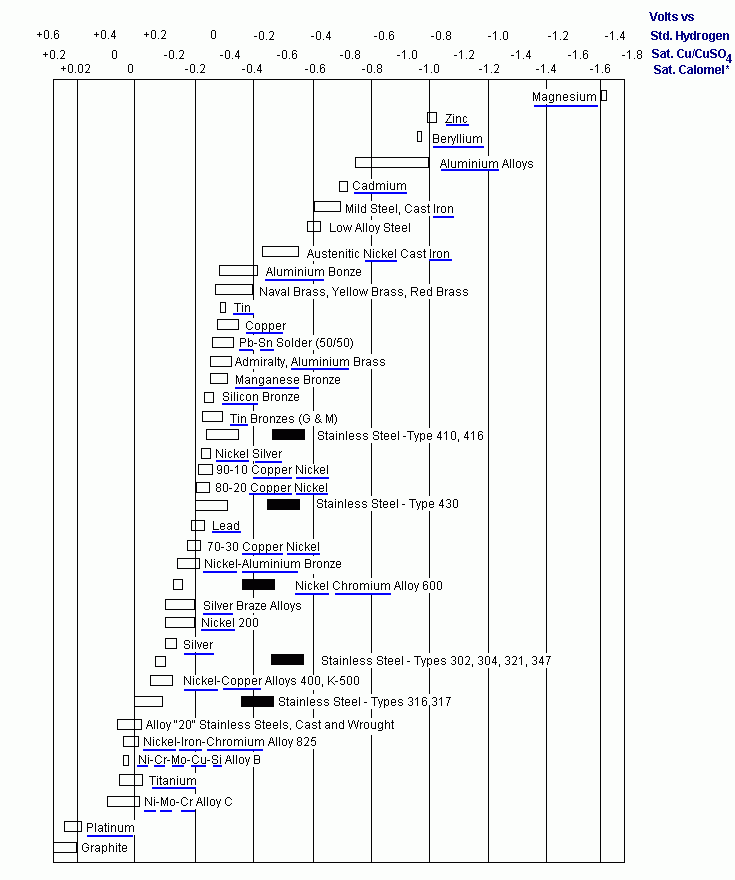
In all of these cases it is the least noble metal on the galvanic scale, or in other words the most reactive metal that will be affected by the corrosion. This can work in your favour or against you. Two cases in point:
- A brass hull fitting is more noble than a steel hull, and will cause the hull to be corroded.
- A zinc anode attached to the hull (make sure there is a good electrical connection to the hull) is more reactive than the hull, the propeller, or any other exposed metals. Therefore the zinc will be quickly destroyed by corrosion and the hull will be protected.
Below is a "galvanic series" chart which shows the various alloys and their position in the series. Alloys to the left will cause alloys to the right to corrode, while the alloys to the left will be protected.
Stray Current Corrosion
Avoidance
The methods to avoid corrosion include:
Exclusion of Water from Electrical Contacts
- Tinned cable.
- Insulation.
- Liquid electrical tape.
- Heat shrink.
- Drip loops.
Cathodic Protection (Zinc)
As can be seen from the galvanic chart above, zinc as a metal is much more reactive than most metals used in shipbuilding. In fact it is the most reactive metal other than magnesium and a few others not shown on the chart, however neither magnesium nor the other metals are suitable for use as a protection method due to the fact that they cause over-protection, which leads to alkali corrosion of the metal you are trying to protect.
(In fact magnesium is suitable for use as a protective anode, however only in fresh water).
So in simple terms, cathodic protection involves turning the metal that you want to protect into a cathode of an electrical cell, while the zinc (also in the water) becomes an anode. In effect you encourage the galvanic corrosion of the zinc and in exchange you will prevent galvanic corrosion of the protected metal.
In order for this to work, the zinc and the metal you are trying to protect must be:
- In the same body of water -- zinc on the outside of your boat will not protect metal fittings in a wet bilge; and
- Electrically connected, outside of that body of water. For example, the zinc should be welded to a steel hull, or in the case of protecting thru-hulls in a fibreglass or wooden boat, connected electrically using a bonding circuit in the inside of the boat to the metal that it is there to protect.
There are many ways of sizing zinc anodes for a particular sized hull but the most common involves a simple formula involving the length (and wetted hull area) of the boat which will give you a size in kg of the zinc anode requirements.
Install Bonding Circuits
"Bonding is the practice of electrically tying together major metal objects on a boat (e.g. rigging and chainplates, engine and propeller shaft, stove, metal fuel and water tanks, fuel deck-fill fittings, metal cases on electrical equipment, etc.) and connecting them to the boat's ground." (Calder p212).
A good diagram of a bonding circuit is in Calder p212, with an electrical schematic on p213. Note that quite heavy wire gauges are used, typically 6AWG or so (for those more used to dealing with metric wire sizes an AWG to European standard wire gauge chart is on Calder p167). The idea being that the bonding circuit is a "husky, low resistance circuit with electrically tight connections" and that any stray currents that may cause galvanic corrosion will choose the path of electrically least resistance to ground, that is the bonding circuit rather than any metal fittings on the surface of the boat.
Water and Oil Exclusion
Especially on a steel boat, it is very important to try to exclude water from the inside of the hull, notably bilges, etc. Anywhere water can pool inside a boat is a potential source of rust and corrosion.
Coating Systems
Steel hulled boats offer special challenges when it comes to corrosion. In addition to having suitably sized sacrificial zinc anodes, offering internal and external protection, excluding water and oil from the bilges, a good coating system is needed.
Coating systems come in many different types and grades from all sorts of different manufacturers, but the basic principles are the same. To best protect my steel boat I use the following:
- A single rust-penetrating and sealing barrier coat (sometimes called a pre-prime), and I always find that the two pack systems are the best. Although there are many different single pack formulas around, and I could name a few "miracle rust cures" but I'm sure we've all seen the advertising, they are generally rubbish.
- Two coats of a two-pack epoxy surfacer. This could be a high-build surfacer for uneven areas or a general purpose two-pot primer and undercoat. Two-pack epoxy gives the best general coating and sealing properties, however it is generally affected by UV light so needs a top coat.
- Two coats of a two-pack polyurethane topcoat.
This is by no means the cheapest solution but if you are the owner of a steel boat you will find that rust is your worst enemy and the best system you can manage to keep it at bay will repay the cost many times over.
Single pack systems are available that at least claim to meet most of the criteria for coating systems, however they have the following limitations:
- They are never as effective as a two-pack system. Two-pack epoxy or polyurethane paint sets by the effect of a chemical reaction causing polymerisation of the paint. Single pack systems are dissolved in solvent, which evaporates causing the setting process to occur. The evaporation of the solvent can leave microscopic pores in the paint surface, leading to rust occurring.
- A two-pack system may never be painted over the top of a single pack system. This is because of chemical reactions that can occur between the incompatible surface layers.
Two-pack systems have their own limitations, however, which need to be noted:
- There is a trade-off between usability and effectiveness. This can manifest itself in a number of ways. For example, the barrier coat (often a transparent layer that may dry to a light red or pinkish hue) often has a long drying time -- overnight or longer. There are "quick dry" formulations of barrier coats, however the faster a barrier coat dries the less effective it will be in penetrating and locking off any surface rust.
- Epoxy systems come in various grades, from high-build (where a thick coat is applied) to a thinner formula. The thicker the formula, the more difficult it will be to apply and the more waste is had when spraying. Thicker coats are often best applied by brush.
- Polyurethane topcoats are highly UV resistant, whereas epoxy coats are generally not UV resistant. However the polyurethanes do not generally have as good a barrier resistance effect as the epoxy coats. This is why it is advisable to put a polyurethane coat over the top of an epoxy coat. Make sure that the coats are chemically compatible -- contact the paint manufacturer if in doubt.
- Polyurethane topcoats often come in a thicker variety which is generally best applied by spraying, and a thinner "brushable" version. Of course you won't get as good a texture by brushing as you will by spraying, but it is most important to choose a brushable polyurethane if you are brushing.
- Feel free to ignore any manufacturer's advice about a polyurethane topcoat's ability to mask the colour of an underlying epoxy layer. Generally, it won't, and at worst it will allow the epoxy colour to bleed through. I have had salesmen assure me that a white topcoat will be perfectly OK applied over a red or grey undercoat (generally a story told when they are out of white undercoat) however this is baloney. So these days I apply one layer of a pink, grey, or buff primer, followed by a layer of white two-pack epoxy, and a white two-pack polyurethane over the top of that.
Testing
Silver/Silver Chloride Half Cell
References
- Nigel Calder, Boatowner's Mechanical and Electrical Manual: How To Maintain, Repair, and Improve Your Boat's Essential Systems — 3rd Edition.
- Miner K. Brotherton, The 12-Volt Bible for Boats — 2nd Edition
Forum Discussion Topics
Links to discussion threads on the CruiserLog forum:
Links
- Corrosion, zincs and bonding - An excellent article (pdf).
Also See
| |
|---|
| | HOMEPAGE | Wiki Contents | |
.